A New Era in Clinical Trials
Current industry challenges present an opportunity for innovation and the adoption of new solutions, particularly in addressing barriers related to patient recruitment, engagement, and retention.
The COVID-19 pandemic opened the door for some significant and noteworthy changes to the way our industry could accomplish clinical trials with a forced mandate to decentralize access and services for the continuation of studies.
While COVID-19 provided a gateway for the launch of an unprecedented level of decentralized clinical trial (DCT) services, what we have seen since is a reevaluation of how DCTs should be operationalized. A retraction in the use of services allowed under the FDA variance, corporate restructurings, staff reductions at eClinical technology and service companies, and staff reductions at CROs all demonstrate this shift. We have also seen a significant decrease in the number of sites running clinical trials, thereby exacerbating the existing challenges in recruiting patients.
This stress upon the clinical trials industry might be the perfect pivoting point for committing to innovation and the adoption of new solutions for sites and patients. Decentralized clinical trials have found their way into the spotlight, and with that comes the ability to quickly identify operational gaps and further industry opportunities.
The pivotal opportunity
Challenges, opportunities, and possibilities remain focused on the adoption of creative solutions to enrollment barriers, which include patient recruitment, patient engagement, and patient retention. These factors all impact successful patient enrollment and expanded patient access. Obviously, the key focus here is the patient, so the solutions mandate both the “human touch” of medical care and leveraging what’s possible using digital technologies to maximize connectivity associated with patient engagement.
The solutions to these challenges and the pressures they create have existed for a long time, and now we have industry ownership in the form of the roles that take responsibility for the operationalization of digital technology, DCTs, and innovation.
Immediate opportunities and possibilities
Sponsors now have increased regulatory pressure to set enrollment goals for a full range of diverse populations in order to obtain relevant pharmacological and genomic data. They also must have patient retention strategies, which may involve collaboration with community-based health professionals supported by the appropriate technology. These pressures can be alleviated by implementing options that are already in place. Yes, these solutions already exist; they just need industry acceptance and adoption.
What are the solutions that can support a more diverse patient population and retention of these patients? There are three major strategic initiatives that are available to implement now:
Strategic initiative I: accessing the global population of mobile device users
The first solution is recognizing that the greatest “new” opportunity for patient recruitment, enrollment, and engagement is tied to accessing the global population of connected mobile device users. And the message that racially diverse populations are not connected is false.
My professional career has included direct involvement in disruptive innovations in the mobile cellular industry, from the early days of analog mobile phones that could only accomplish phone calls to the fully digital devices we have today. I maintain access to important industry reports on the penetration of smartphones and mobile connections, and here are important facts that directly speak to this opportunity:
- Global smartphone users increased by 49.89% between 2016 and 2022 with only 3.668 billion smartphone users, which is 49.40% of that year’s global population.
Advertisement
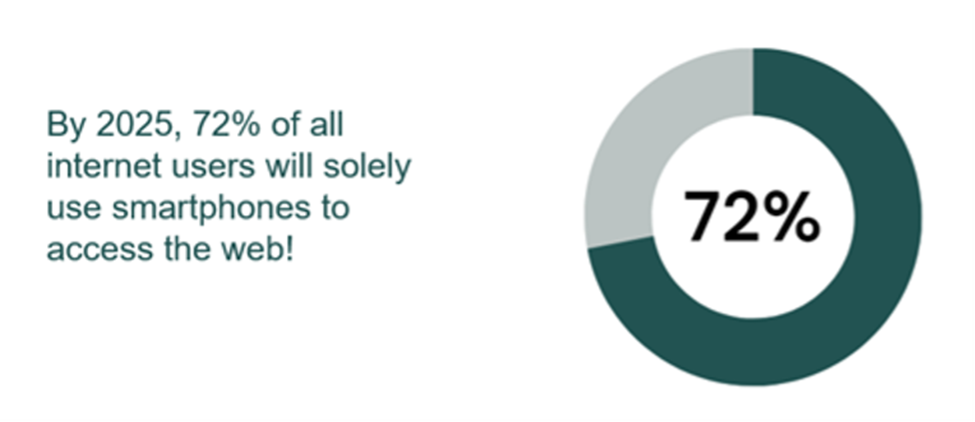
- By 2026, mobile device users will increase to 7.516 billion.
- Today, there are 7.33 billion mobile phones in the world, approximately 90.93% of the global population of 8.01 billion. And of that total, 6.92 billion are smartphone users, which represents 85.74% of the world population.1,2
The call to action for strategic initiative I
One of the biggest challenges today for individuals who have a medical condition is trying to find a clinical study when it is of interest and necessity. A friend of mine shared with me that his wife was diagnosed with Stage IV pancreatic cancer and asked me to help him find a study that might save his wife’s life. Unable to quickly find the right information and have it readily available to me, I feel our industry failed him and his wife. Why is finding a study so challenging?
Why don’t we, as an industry, develop one universal mobile app installed on every mobile smartphone that helps patients find a clinical study? We know the disease we are trying to cure or mitigate. We know the characteristics of the patient desired for the study, i.e., the inclusion and exclusion criteria. We know from the metadata associated with the patient’s location how close they may be to a research site. What a powerful impact an integrated app could have on patient recruitment!
From this universal app, we could help patients identify opportunities for participation, and through the vetting process, could dialogue through SMS messaging and then possibly video chat. The possibilities are there for doing something significant.
Strategic initiative II: maximizing the human touch in operationalizing DCTs
The second solution is focused on the human touch in operationalizing DCTs and technology adoption in bringing patient participation into their community, whether that is in their homes or perhaps at their school, work, church, or another convenient place for their medical assessment and treatments during their clinical trial journey. These solutions go beyond ePRO and eDiaries, which have proven to be vital methods in providing insights on any symptoms and side effects patients may have encountered during their participation in a clinical trial.
Providing patients with the option to have medical assessments and treatments done in their community is an empowering motivation for study participation. The industry perception is that deploying DCT elements, like in-home visits, is just an additional cost to a clinical trial. However, post-pandemic, published results show a positive impact to ROI and benefits to patient recruitment and retention, thereby reducing the timeline for trial completion.
Recent publications have found “substantial value from employing DCT methods in phase II and phase III trials. If we assume that DCT methods are applied to both phase II and phase III trials the increase in value is $20 million per drug that enters phase II, with a seven-fold ROI.”3 And a DCT model provided almost 50% reduction in final protocol to patient in (FPI) timelines compared to a traditional model, as well as over 75% reduction in First Patient-In to Last Patient-In timelines and almost 40% reduction in screen failures.4
The call to action for strategic initiative II
So, the challenge is how do we, the industry, implement the benefit of in-community/in-home trial support services and the human touch that comes with this use as a primary consideration of protocol design where possible, knowing the benefits to patient recruitment, patient retention, and the improvements to study timelines and ROI?
Additionally, in-community/in-home services are supported by a new generation of advanced and portable medical devices that have been developed and can be used in a patient home with a healthcare professional (HCP), replicating what is typically only collected at the site. For example, there are new portable ultrasound imaging devices, portable rapid blood diagnosis devices that can accomplish a complete CBC panel in less than five minutes through a single finger prick of blood versus collecting vials of blood to be sent to a central lab, digital auscultation recording of heart and lung sounds, and even 6-lead and 12-lead EKG devices. All these devices can seamlessly share their data over the cloud to the site. We can move this type of data collection outside of the site and into the patient’s home.
The adoption and use of telemedicine, as we have seen in our own engagement with our Primary Care Provider, adds new opportunities for the patient to communicate with the site PI witnessed or led immediately should there be an event of concern.
Strategic initiative III: the commitment and adoption of digital technology application in studies
We are seeing evangelization in the use of wearables to collect valuable insights into patients’ health. While the industry has been reticent to fully use wearables, concerned with the accuracy of sensor data, there should be an appreciation that wearables, a digital version of an ePRO, are valuable in ensuring consistent measurements and tracking changes over time.
As an example, a patient might be in an osteoarthritis (OA) study and completing industry recognized validated instruments, e.g., the Visual Analogue Scale (VAS) and the Short Form SF-36. These valuable patient data responses are subject to the patient’s feelings and other influences at that moment. Maybe the patient had a cold that influenced his responses. Maybe the patient had a terrible night’s sleep at his daughter’s home after playing with his granddaughters, and that influenced his responses. Maybe the patient wanted to create the image that he was Superman, reporting no issues or pain, and that influenced his responses. How absolutely true are the responses? If the study integrated a wearable, one could look at measurements like steps, range of motion, heart rate, and sleep quality to assess possible biases in the patient’s responses. And the assessment is more focused on changes in measurements versus an absolute number.
The call to action for strategic initiative III
There are opportunities to speed up identification eligible patients and recruitment through the incorporating electronic health records, integrating a patient’s everyday care with what is going on in a clinical trial. This is an opportunity to communicate a patient’s health and care outside of a trial that may impact the results of the trial.
Some of the most exciting developments and biggest hopes are the advances being made in the use of artificial intelligence (AI) in modeling in the drug development process and in the management and analysis of the tremendous volume of patient data that flows into a study.
Full adoption of technology has a long way to go, but there are many opportunities we can take advantage of today. Approaching trial design with a holistic lens will not only identify gaps that technology cannot fill but also create impactful efficiencies for an entire drug program. Integrating the in-person decentralized trial by conducting clinical visits in the community or in the home, paired with the right technology, will be the way forward for clinical trials, as there is now a clear path to successful return on investment. To get the most value from this holistic approach, we need to be designing, developing, and publishing study protocols that focus on maximizing all decentralized trial elements.
Edward Triebell is the Executive Director of Digital Health at Medical Research Network
Category: Uncategorized

